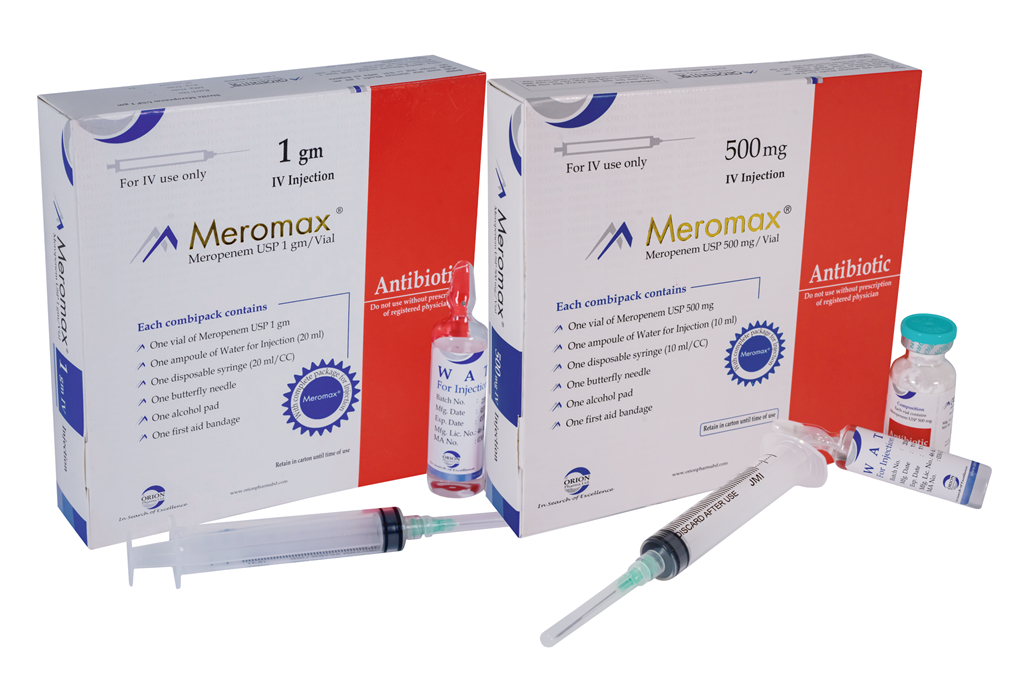

Meromax 500 mg IV Injection: Each vial contains dry substance equivalent to Meropenem USP 500 mg accompanied by a solvent ampoule of 10 ml Water for Injection BP.
Meromax 1 gm IV Injection: Each vial contains dry substance equivalent to Meropenem USP 1 gm accompanied by a solvent ampoule of 20 ml Water for Injection BP.

USP

Carbapenem Antibiotic

Meromax Injcection is indicated for the treatment in adults and children of the following infections caused by single or multiple bacteria sensitive to Meropenem.
Pneumonias and Nosocomial Pneumonias
Urinary Tract Infections
Intra-abdominal Infections
Gynaecological Infections, such as endometritis and pelvic inflammatory disease
Skin and Skin Structure Infections
Meningitis
Septicaemia
Empiric treatment for presumed infections in adult patients with febrile neutropenia, used as monotherapy or in combination with antiviral or antifungal agents.
Other polymicrobial infections

Meromax Injection is contraindicated in patients who have demonstrated hypersensitivity to this product.

The dosage and duration of therapy shall be established depending on type and severity of infection and the condition of the patient.
The recommended daily dosage is as follows:
In the treatment of pneumonia, UTIs, gynaecological infections such as endometritis, skin and skin structure infections- 500 mg IV every 8 hours
In the treatment of nosocomial pneumonias, peritonitis, presumed infections in neutropenic patients, septicaemia- 1 g IV every 8 hours
In cystic fibrosis- doses up to 2 g every 8 hours
In meningitis- 2 g every 8 hours
As with other antibiotics, particular caution is recommended in using Meropenem as monotherapy in critically ill patients with known or suspected Pseudomonas aeruginosa lower respiratory tract infection. Regular sensitivity testing is recommended when treating Pseudomonas aeruginosa infections.

Dosage should be reduced in patients with creatinine clearance less than 51 ml/min, as scheduled below:
Creatinine clearance 25-50 (ml/min) 1 unit Dose (based on unit doses of 500 mg, 1 gm, 2 gm) Frequency every 12 hours
Creatinine clearance <10 (ml/min) 1/2 unit Dose (based on unit doses of 500 mg, 1 gm, 2 gm) <span class="\"Apple-tab-span\"" style="\"white-space:" pre;\"=""> Frequency every 24 hours
Patients with Hepatic Insufficiency
No dosage adjustment is necessary in patients with hepatic insufficiency.
Elderly Patients
No dosage adjustment is required for the elderly with normal renal function or creatinine clearance values above 50 ml/min.
Children
Infants under 3 months
Efficacy and tolerability in infants under 3 months old have not been established; therefore, Meromax Injection is not recommended for use below this age.
Children over 3 months
Over 3 months to 12 years - 10 to 20 mg/kg every 8 hours
Children over 50 kg weight, adult dosage should be used
4 years to 18 years with cystic fibrosis - 25 to 40 mg/kg every 8 hours
In meningitis - 40 mg/kg every 8 hours
There is no experience in children with altered hepatic or renal function.

As with all beta-lactam antibiotics, rare hypersensitivity reactions have been reported. Before initiating therapy with Meropenem, careful inquiry should be made concerning previous hypersensitivity reactions to beta-lactam antibiotics. If an allergic reaction to Meropenem occurs, the drug should be discontinued and appropriate measures should be taken.
Use in infections caused by methicillin resistant staphylococci is not recommended.
The co-administration of Meromax Injection with potentially nephrotoxic drugs should be considered with caution.
Pregnancy category B. Animal studies have not shown any adverse effect on the developing fetus, Meromax Injection should not be used in pregnancy unless the potential benefit justifies the potential risk to the fetus.
Lactation
Meropenem is detectable at very low concentrations in animal breast milk. Meromax Injection should not be used in breastfeeding women unless the potential benefit justifies the potential risk to the baby.

Probenecid competes with Meropenem for active tubular secretion and thus inhibits the renal excretion of Meropenem. This led to statistically significant increases in the elimination half-life (38%) and in the extent of systemic exposure (56%). Therefore, the co-administration of Probenecid with Meropenem is not recommended.
A clinically significant reduction in serum Valproic Acid concentration has been reported in patients receiving carbapenem antibiotics and may result in loss of seizure control. Although the mechanism of this interaction is not fully understood, data from in vitro and animal studies suggest that carbapenem antibiotics may inhibit Valproic Acid glucuronide hydrolysis. Serum Valproic Acid concentrations should be monitored frequently after initiating carbapenem therapy.

Meromax 500 mg IV Injection: Each combipack contains one vial of Meropenem USP 500 mg accompanied by one ampoule of 10 ml Water for Injection BP with a 10 ml disposable syringe, a set of butterfly needle, an alcohol pad and a first aid bandage.
Meromax 1 gm IV Injection: Each combipack contains one vial of Meropenem USP 1 gm accompanied by one ampoule of 20 ml Water for Injection BP with a 20 ml disposable syringe, a set of butterfly needle, an alcohol pad, and a first aid bandage.

Powered by Soft & Tech
© 2003 - 2026 Orion Pharma Ltd. All rights reserved.